TE-1182 is an ADC developed using the CHO-TEM technology platform jointly established by CHO Pharma and T-E Meds.
Its antibody component consists of a full-length IgG molecule, specifically trastuzumab (TRZ), which is produced and glycosylated by CHO Pharma. TRZ is a humanized anti-HER2 IgG monoclonal antibody designed to precisely targets cancer cells that overexpress the HER2 gene on their surface.
Leveraging site-specific conjugation technology, this IgG can be conjugated to 4 drug bundles developed by T-E Meds. Each drug bundle carries two distinct cytotoxic molecules, MMAF and Exatecan (EXT), meaning that each TRZ antibody is conjugated to 4 molecules of MMAF and 4 molecules of EXT. When TRZ specifically targets cancer cells, the cytotoxic molecules are released from the drug bundles, effectively destroying the cancer cells.
Notably, TE-1182 is a homogeneous ADC product with a defined drug-to-antibody ratio (DAR) of 8, exhibiting superior stabilities in vitro and in vivo.
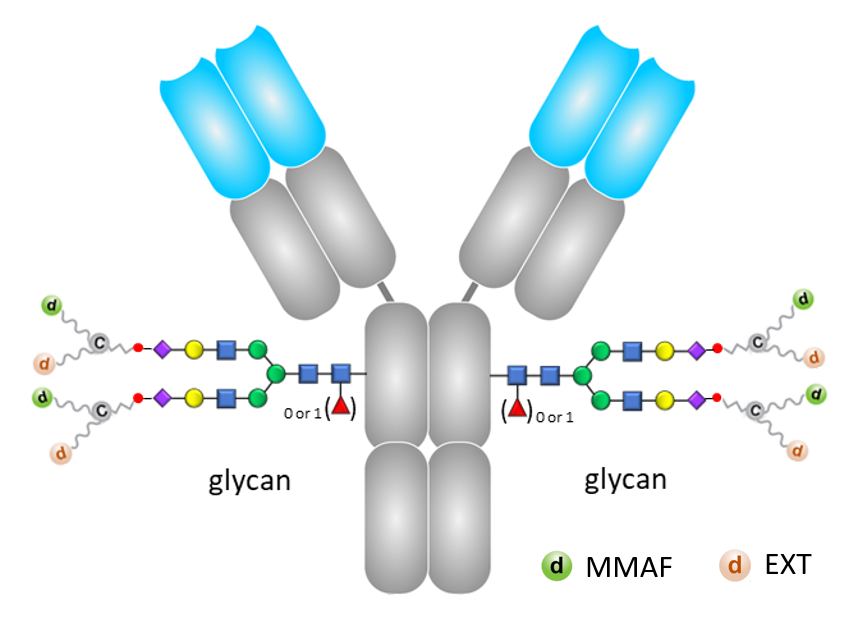
The molecular structure of TE-1182.
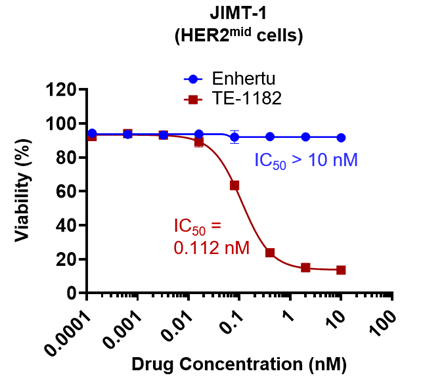
An Enhertu-insensitive cell line (JIMT-1) was used to evaluate the efficacy of TE-1182. TE-1182 demonstrated potent activity against JIMT-1, with an IC50 value of 0.112 nM, whereas Enhertu showed no effect on reducing cell viability at the tested concentrations.
These results highlight the superior efficacy of the dual-drug ADC in targeting drug-resistant tumor cells.
